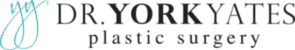
What is ALCL?
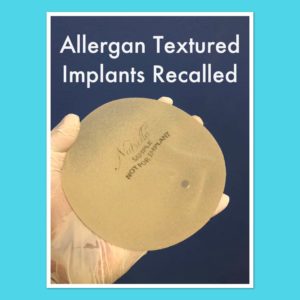
BIA-ALCL (Breast Implant-Associated Anaplastic Large Cell Lymphoma) is a very rare cancer of the lymphatic system. It is NOT breast cancer but rather a cancer of the tissue or fluid that surrounds a breast implant. Ongoing research is underway to help further understand and characterize this disease process. The disease ranges from benign fluid around an implant (seroma) to invasive metastatic lymphoma.
what are the symptoms of aLCL?
Most cases of ALCL present with a late seroma, which is swelling of the breast years after surgery. The average time of presentation is 8 years after implantation but it has been seen from 2-28 years later. It is important to point out that the vast majority of seromas that occur after breast augmentation are not related to seromas. From personal experience, I have seen and tested fluid around twenty seromas developing months to years after augmentation with textured implants and none of these have been related to ALCL. Most cases of seroma after textured implants are specifically related to capsule adhesion, trauma, and inflammation. ALCL can also present with a mass or lump in the breast or armpit and although they require adequate workup, the majority of these symptoms are benign as well.
I have breast Implants, Am I at risk of ALCL?
Current information would suggest that ALCL is entirely related to textured breast implants. To date, there has not been a case of ALCL in a patient with only smooth implants. Most implants placed in the U.S are smooth implants and if you have smooth implants, it appears there is no risk of developing BIA-ALCL. If you do have textured implants, not all texture is created equally and the risk of ALCL can be stratified by the type of texture, which is related to the brand of the implant. In the U.S., the majority of cases of ALCL are associated with the Allergan BIOCELL textured implant specifically, which was the cause of the recall.
What is texture on implants and why is it used?
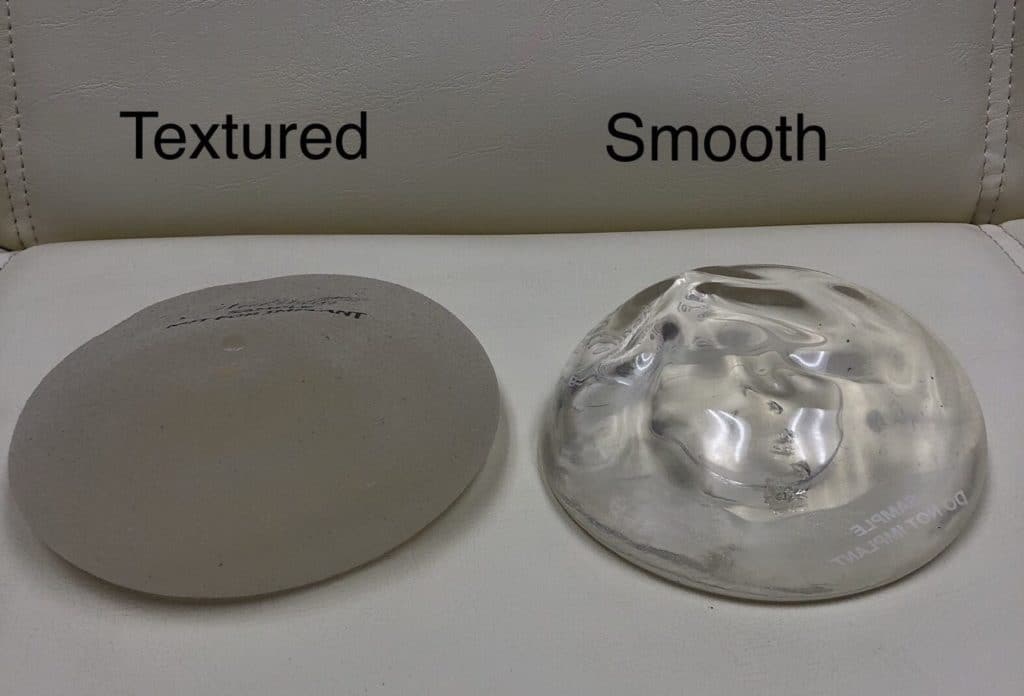
Breast implants can be either smooth or textured. Implants were first texturized in the 1970s as a method of reducing the risk of capsular contracture. Capsular contracture is the most common complication after breast augmentation and it has been shown that textured implants have a lower rate of contracture than smooth implants. Plastic surgeons have also seen a lower risk of implant migration with textured implants, particularly bottoming out and implants moving to the sides. My own personal experience follows that of many other U.S. plastic surgeons. When I started my practice in 2003 only saline implants were available as there was a moratorium on silicone implant sales while they were being studied for safety. Textured saline implants have a high risk of rippling and rupture and most U.S surgeons used smooth saline implants at that time. When silicone implants received approval in the U.S., it was a natural transition to use smooth silicone implants. Then teardrop-shaped implants were approved for use in the U.S in 2012. As teardrop-shaped implants are textured by design, many plastic surgeons who were using only smooth implants became experienced in textured implants and liked them. Speaking for myself, I was quite pleased with textured implants initially as the “grip” of the implant allowed better stability in position. I used a mix of smooth and textured implants from 2012 until 2018 when we started hearing increasing news and concerns about a rare disorder associated with breast implants that we now know as BIA-ALCL. Fortunately, I was using primarily Sientra textured implants at that time and have used very few Allergan textured implants. I have essentially stopped using textured implants at all in the past year out of an abundance of caution.
How do I know if my Implants are the Allergan BIOCELL implants that have been recalled?
You may have received an implant card from your plastic surgeon.

Here is an example of a photo of an implant card I received from a patient yesterday asking this very question. As you can see this is an Allergan implant, specifically, this is a Natrelle Inspira Allergan implant. She was reassured however that the “S” in the “SRF” on the top left stands for SMOOTH. If this were a “T”RF it would be a Biocell Textured implant and cause for concern. The “R” stands for responsive and is the type of gel and the “F” is the profile. Only the first letter is related to the surface characteristics. BIOCELL has been used in both saline and silicone implants. The saline implants that would have BIOCELL are style 163, style 168, style 363 and style 468. The silicone impalnts that would have BIOCELL are style 110, style 115, and style 120 in addition to any Natrell Inspira implants with a “T” in the style type.
If you no longer have your implant card and are not certain what your implants are, your plastic surgeon should have that information for you.
What if I have a textured implant that is not Allergan?
Although the vast majority of cases of ALCL have been associated with the specific type of texture found in Allergan textured implants, that is not to say that the other textured impalnts are completely without risk. Based on current data the risk can be classified by the type of texture as follows:
- Grade 1 (smooth only) – at the time of this writing there have been no cases of BIA-ALCL in smooth only cases.
- Grade 2 (Microtexture such as Mentor and Sientra Textured implants) – 1:82,000
- Grade 3 (Macrotexture such as Allergan BIOCELL) – 1:3,200
- Grade 4 (Polyurethane – not approved in the U.S. market) – 1:2,800
Although extremely rare, there have been cases of ALCL with both Sientra and Mentor textured implants. The FDA has not requested any action be taken on these implants but it is important that patients with any textured implants present concerning signs to their physician.
Have there been any deaths associated with ALCL?
As of July 2019, the FDA has reported there have been 573 world-wide cases of ALCL including 33 deaths since the disease was first reported 20 years ago. Of the 573 cases of ALCL 481 have been known to have Allergan breast implants. In many of the other cases, the implant brand was unknown. Of the 33 cases that resulted in death, 20 of them had an unknown implant brand. Of the other 13 cases that resulted in death, a textured Allergan implant was known to have been placed in 12 of the 13.
What is mY risk of dying from textured breast implants?
Based on this data the lifetime risk of dying from BIA-ALCL is:
- Grade 1 (smooth implants) – zero
- Grade 2 (Microtexture such as Mentor and Sientra Textured implants) – 1:1,423,818 = (1:82,000 x 33:573)
- Grade 3 (Macrotexture such as Allergan BIOCELL) – 1:55,563 = (1:3,200 x 33:573)
ALCL is a very real and serious problem but I can tell you firsthand that patients are experiencing unhealthy panic with this recall and it would be wise to step back and to logically look at the statistics. If you have ever read Freakanomics you will appreciate that we as humans do a terrible job of assessing and assigning risk to our world. The National Safety Council created a chart demonstrating the risks of dying from many different causes. The risks don’t always logically follow our fears about dying. Again, I don’t want to downplay how serious the implant manufacturers, FDA, and plastic surgeons are taking this disease. For perspective, the lifetime risk of dying from ALCL from a BIOCELL implant is a little higher than the lifetime risk of dying from lightning. If you ignore the warning signs of either the risks go up. Wise individuals don’t go outside in a lightning storm. Wise individuals don’t ignore swelling of the breast around a textured implant.
Should I have my textured implants removed prophylactically.
In the event of a seroma or other indication for surgical exploration, I would recommend changing textured Allergan implants to smooth. The tough question is whether it is worth the small but real surgical risk to exchange textured implants if there are no symptoms to help prevent future disease. In my opinion, this is similar to the discussion about prophylactic mastectomy. Women have a 1: 8 lifetime risk of developing breast cancer and 1: 95 lifetime risk of dying from breast cancer (unrelated to implants). A prophylactic mastectomy would eliminate most of this risk but at what cost cosmetically? Every patient needs to decide for themselves if implant removal or exchange to smooth is worth the potential cosmetic changes. For example, a woman with teardrop-shaped textured implants would have to change to a smooth round implant as teardrop implants are always textured. This may change the appearance of the breast, making it appear less natural. For a woman with textured round implants, the cosmetic changes would be expected to be less dramatic.
It is my opinion that any woman with enough concern about their Allergan textured implants to justify changing them should do so. For Sientra and Mentor textured implants I would be less inclined to recommend removal or exchange. Implant exchange is a straightforward operation and you may find that your plastic surgeon is willing to offer the procedure at a significant discount. We certainly are. We are also actively working with Allergan and there are rumors that they may soon provide new implants free of charge for some of those patients.
Fortunately, in my career, I have used very few Allergan textured implants. We are in the process of identifying and reaching out to all of those patients to make sure they are informed of the situation. If you are a patient of ours and have lost your implant card and are uncertain of which implant you have, feel free to reach out to me directly through our contact page.
What are the FDA Recommendations if you have Allergan BIOCELL Breast implants?
- If there are no symptoms they are not recommending removal. This is a recall of unused inventory. Not a recall of implants that have already been placed.
- Know the symptoms of BIA-ALCL, primarily pain, and swelling around or near the implant.
- Express symptoms or changes with your doctor.
- Patients with confirmed BIA-ALCL need treatment including implant and capsule removal