Table of Contents:
Please click on any of the bullet points below to jump to the section of your choosing!
- What Is Breast Augmentation Surgery?
- How Is Breast Augmentation Performed?
- Breast Augmentation Results
- Am I A Candidate For Breast Augmentation?
- What Should I Expect From The Recovery From Breast Enhancement?
- What Is The Difference Between Saline And Silicone Breast Implants?
- Saline Implants
- Silicone Implants
- Breast Augmentation Faqs
- Breast Implant Faqs
- Breast Augmentation Reviews
- Breast Enlargement Surgery Video
What is Breast Augmentation?
Breast augmentation is our most commonly performed procedure, and we take great pride in our breast enhancement outcomes. In Utah, breast augmentation is a very popular procedure as there are a lot of fit, young mothers who have lost shape or support of their breasts after childbearing. Utah plastic surgeon Dr. York Yates serves patients from Salt Lake City, Layton, and surrounding areas, and he uses modern techniques to maximize outcomes while minimizing recovery. Most patients are back to normal activities within a week.
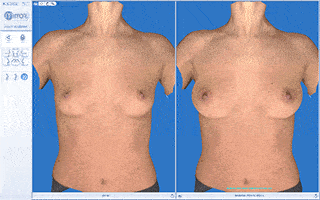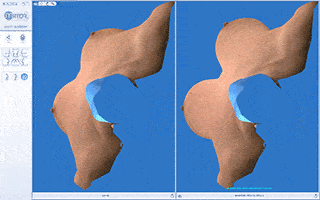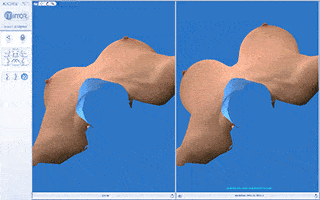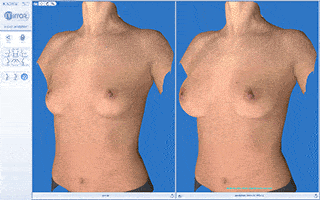
Every breast augmentation patient receives a thorough consultation consisting of listening to goals, implant sizing, and simulation with 3D imaging assuring the most precise implant selection possible.
How is Breast Augmentation surgery performed?

Breast augmentation is a procedure in which a precise pocket is created under the breast to place a breast implant, which adds volume and shape to the breast. There are hundreds of options for the size, shape, and profile of implants; the specific implant chosen will be matched to the patient's body in reference to their size and shape goals.
During breast augmentation, patients are under anesthesia, allowing for a comfortable and easy experience. There are a few anesthetic options for breast augmentation, which can be done under either general anesthesia or local anesthesia with sedation. Dr. Yates's preferred technique is deep sedation with excellent local anesthesia in our AAAASF-certified office operating suite. The patient is sedated and monitored by the anesthetist. The level of sedation is deep (no pain, no awareness), like very deep sleep.
The implant pockets are meticulously developed and compared for size and symmetry. The breast implants are placed, and the incisions closed. The amount of sedation is lightened at the time of closure, and the patient is awake enough at the completion of the procedure to walk assisted to the recovery room. Most patients have basically no pain in the recovery room because of the local anesthetic.
Results of Breast Augmentation

Candidates for Breast Augmentation Surgery
The decision to have breast augmentation surgery is an entirely personal one. Whether to add volume to breasts, restore balance to asymmetrical breasts, or create a more voluptuous figure, there are many reasons a woman may choose to have breast augmentation. Our Utah breast implant patients have a variety of breast size goals that need to be considered. But that decision needs to be for her, and her alone.
Breast augmentation satisfaction ranks among the highest of any cosmetic procedure. Studies show a 98 percent overall satisfaction with their breast augmentation surgery and its aftereffects. It has been the single most popular cosmetic surgery performed in the U.S. since 2006. From a physical standpoint, good candidates should be reasonably fit and near their ideal weight. They cannot be pregnant or currently breastfeeding.
Recovery from Breast Enhancement Surgery

- You will arrive comfortably into our recovery suite
- Our Rapid Recovery techniques will help you recover easier and quicker
- For the first few days you may need pain medications, these are frequently not necessary
- Return to office work and light exercise at one week
- Return to physical work and moderate exercise at three weeks
- Return to full activity at six weeks
Saline vs. Silicone Breast Implants

Saline Implants
- filled with “salt-water”, a more natural material
- filled by surgeon allowing small volume adjustments
- feel less natural
- higher risk of rippling
Silicone Implants
- fill material is silicone and doesn’t disappear if ruptured (silent rupture possible)
- more natural feel
- less rippling
- higher patient satisfaction
Breast Augmentation FAQs
Please click on any of questions below to view their answer!
The cost of breast augmentation surgery in Utah is generally less than in many areas of the country. Utah has a reputation for excellent breast augmentation results at an affordable price. The average cost of breast augmentation in Utah is currently around $7500. We have seen total costs of a Utah breast augmentation range from $5000 to $12,000. Although not an industry standard, we have chosen to list our all-inclusive cost for breast augmentation as well as the costs of other common procedures performed by Dr. Yates.
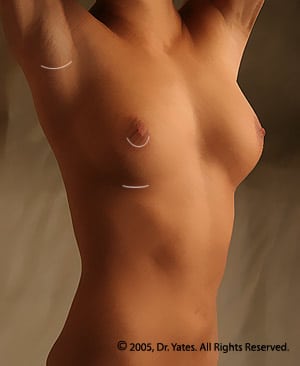
Three incision choices are generally used; around the nipple, beneath the breast, or in the armpit. The incisions beneath the breast or around the nipple are preferred by Dr. Yates, as perfect symmetry of the breast implant pockets is more consistently achieved.
Dr. Yates takes pride in making his scars as small and unnoticeable as possible. There is increasing data that a crease (inframammary) incision has the lowest risk of capsular contracture and has the lowest complication rate. The majority of plastic surgeons, including Dr. Yates, prefer this incision for the majority of breast surgery patients.
Dr. Yates does all of his incision closures internally and uses high-tech knotless sutures to achieve the best scars possible.
Subglandular
- placed above the pectoral muscle
Pros
- less “animation deformity”
- can feel more natural in certain circumstances
- Easier recovery with no concern of future weight lifting or body building
Cons
- higher risk of capsular contracture and poor implant support (bottoming out)
- higher risk of rippling
Summary
- best suited for patients with adequate implant coverage (greater than 2 cm pinch thickness)
- best suited for smaller implants for support and coverage reasons
- NEW - Dr. Yates is LOVING Motiva implants above the muscle. These implants have lower risks of capsular contracture and rippling and perform very well above the muscle for many patients.
Subfascial
- the implant is placed below the fascia that envelopes the pectoral muscle. A modified version of a sublandular pocket, the implant is above the muscle.
- Dr. Yates typically prefers the subfascial position to a traditional subglandular as it provides a little more coverage and support to the implant. The fascia type and coverage is variable from patient to patient.
Pros
- similar advantages to subglandular but a little better support and coverage
- NEW - Motiva implants have a lower rippling and contracture risk compared to other implants and perform well in the subfascial position for many patients.
Submuscular
- the implant is positioned below the pectoral muscle. The lower portion of this muscle is divided in this surgery
Pros
- less implant visibility, particularly in the upper pole
- best support
- lowest risk of capsular contracture
- lowest risk or rippling
Cons
- when flexing the implants distort, “animation deformity”
- higher risk of muscles pushing implants to sides over time
- a little tougher recovery and patients are asked not to "build" their pectoral muscles with submuscular implants.
Summary
- This is the best option for patients with little breast tissue coverage or for patients who desire large or heavy implants.
- Very muscular patients are at more risk of position instability with submuscular implants.
The internal bra is an exciting new concept in breast augmentation and breast implant revision surgery. An internal bra involves adding a synthetic scaffold to further support a breast implant. An internal bra is not helpful or necessary for routine breast augmentation cases but there are situations where it is a nice addition. Click the link if you want to learn more about the “internal bra“.

The perfect breast shape for natural breasts should have a little more breast volume below the nipple than above with nipples tilted slightly upward. Many patients who want breast implants want a greater degree of upper fullness than natural. What you consider your ideal breast shape is based on personal opinions. Sharing these shape goals with your surgeon can help assure you get the outcome you desire.
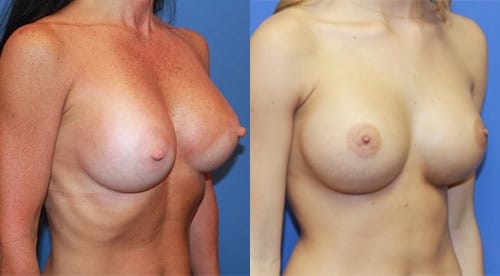
Yes, the breast implant size can be larger in the smaller breast to help improve asymmetry of size. Other tools include fat transfer to the breasts and breast reduction. If there is a significant asymmetry of the shape of the breasts, a breast lift may be required to improve symmetry.
Dr. Yates does a lot of breast augmentation surgery. This is an efficient operation, and there are a few tricks to make it quick and relatively easy on the patient. Rapid recovery methods are always used. The overall surgery time is around 45-60 minutes.
After a full recovery, you should be able to do most everything you were doing prior to your breast augmentation but for submuscular implants may need to limit the intensity of chest exercise. Building the pectoral muscle bulk can cause the implants to push to the sides over time and may require additional surgery to regain support.
Breast augmentation is most commonly performed using implants, but another equally effective method is breast fat grafting, also known as breast fat transfer. In this procedure, fat is harvested from unwanted areas of the body and transferred to the breasts.
The results can be beautiful, but they differ from those achieved with implants. One limitation of fat grafting is the amount of fat that can be transferred; typically, the smaller the breast, the smaller the increase in size will be. To illustrate this volume limitation, Dr. Yates uses the analogy of a sponge and a glass of water.
Additionally, the shape of the breasts after fat transfer can differ from that achieved with implants. Fat grafting often results in a more natural appearance, which may be slightly lower and less full at the top than implant-based augmentations. Whether this is a positive or negative aspect depends on the individual’s goals.
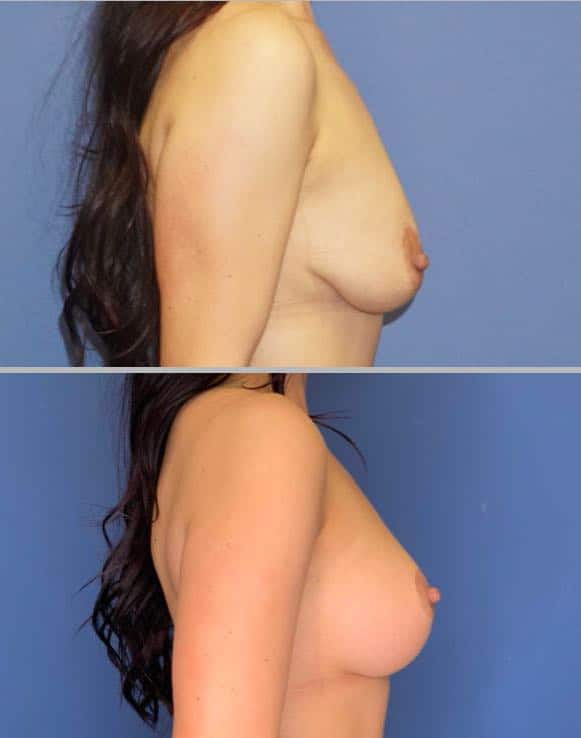
Fat grafting is an excellent option for patients who want larger breasts but are concerned with having breast implants for any reason. It is also a great option for patients who currently have implants and want them removed but desire more shape and fullness than their natural breasts.
For some patients, a hybrid implant and fat transfer procedure is an ideal choice. In this approach, a smaller implant is selected, and additional volume is provided through fat transfer. This technique can enhance implant coverage and softness, allowing for a smaller, more stable implant that results in a more natural appearance.
The cost of natural breast augmentation is often more than that of traditional augmentation depending on how many liposuction areas are desired.
An internal bra or internal lift breast augmentation can mean different things to different plastic surgeons. Most accurately, an internal bra procedure adds additional synthetic support to the breast implant in the way of synthetic material. The most common materials are Strattice and Galaflex. These are structurally different but have in common that they are slowly incorporated and become part of the breast adding lower support like an internal “push up” bra.
These materials are expensive and can easily add $2,000 to $4,000 to procedure costs. There is little evidence that there is a long term benefit from them. Also, there are increased risks associated with their use. These pros and cons need to be weighed when considering an internal bra breast augmentation.
Breast augmentation doesn’t have any effect on a woman’s reproductive system, so it neither increases nor decreases the chances of becoming pregnant.
With pregnancy, implants don’t change but the breasts over them can. These changes in the breast after pregnancy are the same types of changes that can occur in women without implants. Breasts can increase in size during pregnancy and then deflate, leading to droop and volume loss.
Generally yes, but there are exceptions to every rule. Click for more information about breastfeeding with breast implants.
As with any surgery, there are possible risks and complications. As a perfectionist, Dr. Yates takes every precaution to prevent these. Dr. Yates will discuss these at the time of your breast augmentation consultation.
These risks include, but are not limited to, asymmetry, unhappiness with size, implant deflation, implant rupture, implant rippling, capsular contracture, and poor scarring. Generally, these complications require breast implant revision surgery.
Two kinds of breast implant issues called “breast implant-associated large-cell lymphoma (BIA-ALCL)” and “breast implant illness (BII)” have come to light in recent years, although the confirmed cases are very low. BIA-ALCL has been proven to be directly related to breast implants, while BII is theoretical at this point and hasn’t been recognized by the medical community as a definitive diagnosis. Neither issue has changed the FDA’s position on breast implants, but both issues are being closely studied.
Nipple sensation is often impacted immediately following this surgery, with the nipples either being numb or very sensitive. This, because the surgery can disturb some of the nerve pathways. Regular sensation returns in all but a very small percentage of patients. Breast sensitivity should not be affected. There may be some temporary changes, but these will resolve as you heal.
Nothing really, they all describe the same thing. However, “boob job” and “breast jobs” are not generally terms used by professionals.
Yes, rapid recovery breast augmentation techniques are used for every breast augmentation patient, Breast augmentation has advanced tremendously in all aspects including patient recovery.
The key components of rapid recovery breast augmentation are:
Preoperative
- preoperative hydration to avoid nausea
- preoperative room warming
- ERAS protocol (oral medication cocktail)
Intraoperative
- precise pocket elevation in the perfect plane between the pectoralis major and pectoralis minor, a plane with few nerves or vessels
- avoid muscle “tearing” and nerve injury
- avoid any rib trauma
- cauterization of all blood vessels for a bloodless surgery
- long-lasting local anesthetics
Postoperative
- early arm motion
- early walking and general mobility
- a “no narcotic” mindset
There is a period of tightness after breast augmentation that can last days or months. The tightness results from either a tight skin envelope, tight muscles over the implants, or both. The implants never actually harden, it is just the tissue around them that makes them feel firm. The muscle and skin gradually relax over weeks to months as the implants soften. Some refer to this as the process of “dropping and fluffing”.
Breast Implant FAQs
Please click on any of questions below to view their answer!
The history of breast implants is an interesting one. The first implants were placed in the early 1960s and there has been a lot of improvement in techniques and implants since that time.
One of the most challenging and essential decisions for a patient is determining which size of breast implant to use. The degree of breast enlargement is dependent upon the volume of the patient’s breast tissue in addition to the size of the breast implant used.
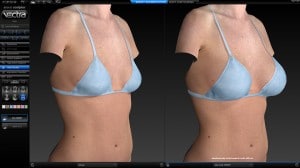
We will use a variety of methods to arrive at the ideal implant volume including “trying on” breast implants of various sizes, dimensional analysis, reviewing photos brought by the patient of “ideal” breast size or aesthetics and 3D imaging.
Vectra 3D imaging is a confirmatory method of breast implant sizing. A 3D image of the body is taken, which can be modified with imaging software. We can simulate and visualize the changes that can be made with a variety of implant shapes and sizes as well as visualize the pros and cons of a breast lift.
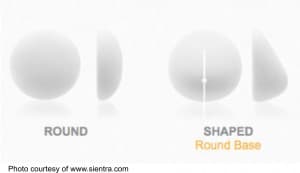
Although we have had great success with teardrop-shaped breast implants, we rarely use them anymore because of a few unique complications that are essentially only seen with textured implants. Of note, all teardrop-shaped implants are textured.
- Seroma
- Implant rotation
- BIA-ALCL – a type of lymphoma associated exclusively with textured implants.
For some breast augmentation patients, these have the advantage of achieving a more natural breast shape. The increased projection in the lower breast can help with slight degrees of breast droop as well.
The ideal candidate for a teardrop implant is interested in a very natural shape and would consider a slightly greater expense and risks an acceptable trade-off. These teardrop-shaped implants are not for everyone, and generally, patients who prefer a “rounded” upper breast are better candidates for round implants.
Breast implants consist of a silastic shell filled with either saline (saltwater) or silicone. Silicone breast implants generally have a more natural look and feel. Silicone implants have a lower risk of rippling, rupture, and generally, have higher patient satisfaction. Saline breast implants are less expensive, more adjustable, and can be placed with a slightly shorter scar.
There is a concern with “silent ruptures” with silicone breast implants, but not with saline implants. A silent rupture occurs when there is a rupture of the implant that is not apparent to the patient. There is some controversy regarding the safety and method of diagnosis of silent ruptures. Current silicone breast implants are cohesive, meaning they are more solid than liquid. Cohesive silicone is much less likely to get into the surrounding tissues than previous implants and typically remains contained in the implant capsule. The trend in the U.S. has been greatly in favor of a preference for silicone implants.
Saline Advantages
- Cost
- Adjustable fill
- Length of scar
- Ease of diagnosis of rupture
Saline Disadvantages
- Higher rupture rate
- Feel firmer and less natural
- More rippling
- Less natural shape
- More likely to displace
Silicone Advantages
- Softer feel
- Less rippling
- More natural shape
- Rupture less common
- More stable in position
Silicone Disadvantages
- Cost
- Less adjustability (pre-filled)
- Length of scar
- Silent rupture is possible
Although differences exist between brands, most breast augmentation plastic surgeons are loyal to one brand or another. Some of us (like Dr. Yates) prefer to use all brands for a more precise implant fitting. There are subtle characteristics of each implant brand that can assist in picking the best breast implant for a given patient, not just one that works. There are four implant brands with FDA approval for use in the U.S.

Mentor Breast Implants
- Full line of saline and silicone implants with many profiles
- Mentor Round silicone implants are called MemoryGel ®, FDA approved in 2006
- Mentor round silicone implants are less cohesive than other brands (soft)
- Mentor round silicone implants have a lower fill ratio than both Sientra and Allergan implants which can lead to a more natural result
- Implant shells thinner than Sientra and Allergan. Less palpable but possibly a little more chance of rupture
- Shaped implants are called MemoryShape ® which was FDA approved in 2013 and currently have five shape options
- Textured implants (Siltex ®) use a stamp manufacturing method and have less tissue ingrowth than Sientra or Allergan but a high coefficient of friction. There are no standardized studies comparing contracture rates and stability over time to know which texture type is best. Suffice it to say that all textured implants have a slightly lower contracture rate and position stability at the expense of a higher rippling rate.
Sientra Breast Implants
- Silicone implants only
- Round and shaped implants FDA approved in 2012
- Distribute ONLY to board-certified plastic surgeons
- Standard silicone is more cohesive than either Mentor or Allergan
- Shell thickness is between Allergan and Mentor and in Dr. Yates opinion is best.
- Textured implant (TRUE texture ™) and between Mentor and Allergan in terms of tissue ingrowth
- Shaped implants come in 5 shape options
- 20 year financial assistance warranty in the event of rupture (best warranty)

Allergan Breast Implants (Natrelle and Inspira)
- Full line of saline and silicone implants with the largest amount of profile and size choices
- Round Allergan silicone implants approved in 2006.
- Shaped implants (style 410) FDA approved in 2013. Removed from the market in 2019 for association with BIA-ALCL.
- Inspira implants approved in 2015. These have a high fill ratio designed to give more fullness and less rippling. Inspire implants to have more size and profile choices than any other round implant. This can be helpful in correcting small degrees of asymmetry
- Textured implants (Biocell ) are manufactured with a “salt loss” technique resulting in very aggressive tissue ingrowth. This may help stabilize the implant position but has a higher risk of peeling the capsule away (double-capsule) and seroma. This Allergan Biocell texture has been implicated in a higher than acceptable risk of ALCL and these were removed from the market in 2019.

Ideal Breast Implants
- FDA approved in 2014
- “Structured” Saline implant that feels more like silicone than a standard saline implant
- More expensive than typical round silicone implants
- The selling point is that there are no silent ruptures as the entire implant is saline-filled
- Limited profiles and sizes
The Ideal Implant is a new saline implant with inner chambers that slows the flow of saline, creating a saline implant that feels a lot like silicone implants. The Ideal Implant® received FDA approval in November 2014. These implants are more expensive than traditional saline implants but have many of the advantages of silicone implants including a more natural feel, and lower rippling risk.
The major advantage of these implants as compared to silicone implants is there is no risk of “silent rupture.” This should be strongly considered in any woman who is very concerned about silicone or silent ruptures. One limitation is in implant sizing. There is only one profile in the Ideal Implant®, which closely matches a high profile silicone implant.
Every patient has about 100 cc range of implant volume that fits their breast dimension well, and these implants may be too narrow in very small sizes for most patients. We are one of a handful of offices that offer the Ideal Implant® in Utah.
Have you ever tried to rupture a breast implant with your bare hands? Of course you haven’t, but we have. Watch these educational, entertaining videos of my staff trying to do just that. Think breast implant tug o′ war.
Incisions made around the areola can sometimes impact breastfeeding, which is one of the reasons Dr. Yates prefers to avoid this incision choice. A more common problem is inadequate breast milk production. Though the milk ducts remain intact and the mother is capable of producing a normal supply of milk, at times nerve damage in the breast blocks the hormonal signals to the brain that triggers milk production. This is a rare problem, however.
As with any manufactured product, breast implants have a lifespan. At some point, the implants will rupture or begin leaking and will need to be replaced. How long will that be? Manufacturers warranty their implants for ten years, and will generally reimburse patients for failure prior to that timeframe. But how long your breast implants will last can vary wildly. You should expect to need to replace your implants every 10 to 20 years. Implant durability is increasing, so this could lengthen. Regardless, if you’re in your 30s, you’ll need to replace your implants at least twice in your life, possibly more than that. Replacement surgery is much easier than the original augmentation surgery, as Dr. Yates can enter through the original incisions.
For patients who want a no-maintenance option, fat transfer to the breast could be considered.
Yes, the manufacturer provides a free lifetime warranty against implant rupture or deflation. They also offer help with the surgical fees of replacement of failed implants for 10 -20 years. Implant manufacturers are also offering very generous limited warranties against capsular contracture.
Saline breast implant deflation is relatively easy to diagnose and correct. The saline is absorbed, and the breast deflates. Deflation generally requires a simple remove and replace procedure.
A silicone breast implant rupture is a little more difficult to diagnose and treat than saline implant deflation. The implant material is not absorbed and can cause hardening and deformity of the breast. It is also harder to correct surgically, as the implant material may need to be removed as well. For more information about the treatment of silicone breast implant rupture click here.
There is no evidence that breast implants have or could ever cause breast cancer. There is a newly identified type of lymphoma, ALCL that has been found in women with breast implants. As of the time of this writing, it has only been found to be associated with textured implants.
In the U.S. most of the cases of ALCL are in patients who have Biocell textured implants, but it has been found in patients with other types of textured implants as well. Although very rare and typically easily treatable with implant and capsule removal, this is a REAL issue and there is ongoing research. I wrote an update about ALCL on my blog.
As of July 24, 2019, Allergan has instituted a worldwide recall of all of their textured implant products. The FDA has not at this point recommended elective removal or exchange of these implants as the risk of developing ALCL is very small. The reason that Allergan, and not the other FDA approved implant manufacturers, has recalled their implants specifically is because their implants are a macrotexture product and are the primary implants involved in ALCL in the U.S.
If you have Allergan textured implants and want to have them exchanged for smooth implants out of an abundance of caution, it is reasonable to do so in our opinion. Certainly, if there are symptoms such as unilateral swelling, this should be examined thoroughly.
Good News. As of July 24, 2019, Allergan will be providing smooth implants to any patient who would like to exchange their current textured Allergan implants. They will not be providing financial support for replacement as the FDA has not recommended the removal of textured Allergan implants without symptoms. Nonetheless, Allergan should be applauded for stepping up and doing the right thing for the patients. Allergan is calling this the BIOCELL Replacement Warranty. This program will run for two years from July 24, 2019, to July 24, 2021. They will provide free smooth implants in the same size as the original implants. This warranty does not cover other revisions or additional surgery for size change. The covered devices include all BIOCELL textured implants which may have also been under the brands McGhan, Inamed, Natrelle, or Inspira. If you are interested in pursuing this claim, we would be happy to do the legwork for you.
We would be honored to have you as our next breast augmentation patient. To schedule a consultation, call the office at 801.525.8741 or if you would prefer an online visit fill out a virtual consultation form. Dr. Yates will respond in a timely fashion to your inquiry.
Breast Augmentation Reviews
“I feel so much more comfortable moving forward with my breast augmentation after consulting with Dr. Yates and his staff. They are all so helpful, and Dr. Yates is very detail-oriented. I know I will be in great hands!”
– Marie P.
“I had such a great experience with the staff and Dr. Yates. I am extremely happy so far with my outcome. Dr. Yates and his staff answered all of my questions, and I was very confident that I would be happy with my results. I highly recommend Dr. Yates to anyone thinking of having breast augmentation or other types of breast surgery. It was a super good experience!”
– Tresa B.
“If I could rate Dr. Yates and his staff 10 stars, I would. From the minute you walk in, the front desk ladies are very warm and welcoming. I recently had a breast augmentation, and I couldn’t be happier with the results. Dr. Yates makes sure to answer and explain every question and concern and is very honest in what to expect. I really appreciated that! I would refer anyone to his office in a heartbeat.”
Breast Enlargement Surgery Video
Call us at 801.525.8741 to schedule a consultation or contact us here to learn more about breast augmentation with breast implants in Layton and Salt Lake City, Utah.